Dissolved oxygen (DO) is oxygen gas (O2) dissolved in water. Gases in the atmosphere such as oxygen, nitrogen and carbon dioxide naturally dissolve in water to some degree. Like salt or sugar, these gases are invisible in water once they have dissolved.
The element oxygen occurs in nature in many forms. Although most people know that oxygen is part of the water molecule, most would be surprised to hear that oxygen is also the most abundant element in rocks. In these forms, oxygen is bound to other elements such as hydrogen, silicon or carbon. Molecular oxygen (O2) found in air is different from other forms because it is not bound to other elements. In nature, the O2 we breathe is chemically much more reactive than the more common forms of oxygen we come into contact with. This allows plants, animals and other organisms to use O2 to metabolize their food through the respiratory process.
Concentration and solubility
The amount (concentration) of O2 dissolved in water is most often expressed in milligrams per liter of water (mg / l). This concentration is called the dissolved oxygen (DO) content of the water. There is a natural tendency for water in contact with air to dissolve O2 until the saturation concentration is reached. For example, the oxygen content in fresh water at 25 ° C in contact with air is 8.3 mg / l, provided that the equilibrium between water and air is reached and nothing removes the O2 from the water.
DO concentrations are sometimes expressed as % of saturation. When the oxygen content of the water is at the saturation concentration, it is said to be 100% saturated. For example, if the oxygen content in fresh water at 25 ° C is 5.0 mg / l, it is 60% saturated (5.0 divided by the saturation level of 8.3 mg / l, multiplied by 100%).
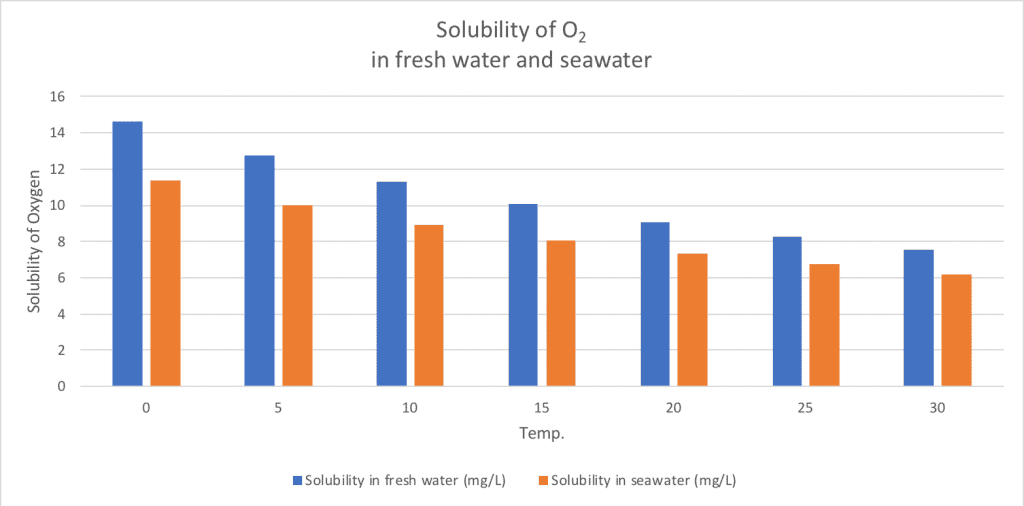
This saturation concentration is known as the solubility of O2, which is the amount of O2 that water can absorb. The solubility of O2 changes with temperature, salinity and pressure. The solubility of O2 in water increases with decreasing temperature, which means that cold water can absorb more O2. For example, cold water at 5 ° C (12.8 mg / l) contains about 55% more dissolved oxygen than warm water at 25 ° C (8.3 mg / l) [1].
Because water temperature varies with the seasons, oxygen levels tend to be higher in the cooler months because the solubility of O2 is higher in cold water. In summer, water levels tend to be lower and the air warmer, resulting in warmer water and lower oxygen levels.
The salinity of water also affects the solubility of O2, so seawater can contain about 20% less O2 than freshwater [1].
Source: [1]
The solubility of dissolved oxygen changes with temperature and salinity.
Pressure also influences the solubility of O2. The water pressure at a given depth depends on the height of the water column above it, so the pressure increases with depth. Water with higher pressure can contain more O2, which means that the solubility of O2 increases at greater depths. For example, water at a depth of 4 m can contain about 40% more O2 than water at the surface [2].
It is possible that water has an oxygen content higher than the solubility of O2 (more than 100% saturation). This condition is called oversaturation and can occur under special circumstances (see below).
Sources and sinks of O2 in water
The main source of O2 in water is the atmosphere. Oxygen molecules slowly enter water at the surface. This process is naturally supported by turbulent flowing water, wind and waves. For this reason, still water tends to have lower oxygen levels than fast-moving water. Natural aeration of water by rapids or waterfalls or artificially by bubbling air through water, turning water wheels, or spilling through dams greatly accelerates the transfer of O2 from air to water. O2 also enters water bodies from tributaries and groundwater discharge.
O2 in water is also produced by photosynthesis, in which plants and algae convert dissolved carbon dioxide (CO2) into organic matter and release O2 into the water. Photosynthesis takes place only at times of day when light is available. The depth at which photosynthesis occurs depends on the clarity of the water. In murky water, the light may not reach the bottom of a deep lake.
Aquatic plants, animals, and microbes consume O2 through respiration, converting organic material used as fuel back into CO2. This is the opposite of photosynthesis. Many people are surprised to learn that plants both consume and produce O2. Plants consume O2 at night through respiration and release O2 during the day through photosynthesis. For this reason, oxygen levels in some aquatic environments tend to decrease at night and increase during the day.
Microbes and fungi also consume O2 by decomposing dead organic matter. This often occurs in deeper layers of the water column as dead material sinks to the bottom. For this reason, deeper water layers often have lower oxygen levels than shallower layers.
DO and water life
Different types of aquatic animals have different DO requirements. Animals that feed at the bottom of a water body, where oxygen concentrations tend to be lower, can typically tolerate lower oxygen concentrations that animals near the surface have. Most fish can survive and grow at oxygen concentrations of 5 mg / l or more, although spawning and optimal growth may require higher concentrations [3].
If the oxygen level is too low for a particular species, the animal may become lethargic or die. Hypoxia is a condition in which oxygen levels are low enough to threaten aquatic species. Hypoxia can cause dead zones in water bodies where fish and other aquatic life are absent. An oxygen level of less than 1-2 mg / l is generally considered hypoxic, and a level of less than 3 mg / l is cause for concern. These levels are below the requirements for spawning and growth of most fish.
At the opposite extreme, oversaturation of water with O2 can cause health problems in fish. Supersaturation occurs when the solubility of O2 in water decreases rapidly or when O2 is produced rapidly by photosynthesis. For example, the solubility of O2 may decrease as water temperature increases, so a rapid increase in water temperature may lead to supersaturation. O2 oversaturation can cause a health condition in fish called gas bubble disease.
Environmental impact on DO
Since most aquatic organisms require dissolved O2, the oxygen content of a water body is often used to assess its health. Oxygen levels in water bodies can be affected by a number of different environmental issues. For example, runoff associated with logging or agricultural waste can carry excess organic material into water bodies, which can lead to O2 depletion as the material decomposes.
Another problem is excessive nutrients that can enter water bodies through runoff from fertilizers on agricultural land or recreational areas (e.g., golf courses) or from wastewater treatment plants. Excessive nutrients can lead to algal blooms, a process known as eutrophication. Algal blooms can prevent light from reaching aquatic plants, and dead algae provide a source of organic matter that can lower oxygen levels as it decomposes. As the dead algae sink, this problem particularly affects deeper water layers and animals that live on the bottom or bed of the water body.
Riparian vegetation (plants that live on the banks of a stream or river) protects the oxygen levels of streams by providing shade that keeps the water cool. However, when this vegetation is removed, the water temperature may increase, resulting in a corresponding drop in oxygen levels.
Water temperature can also be affected by other human activities. When water is withdrawn or stored for drinking, irrigation, or industrial purposes, especially during dry months, stream levels can drop, making them particularly vulnerable to temperature fluctuations and heating. The resulting decrease in oxygen levels can harm aquatic life in these waters. When water is used for industrial cooling processes and then returned to a stream, its temperature is often higher than the water in the stream, causing the stream to heat up and its oxygen content to decrease.
Conclusion
Dissolved oxygen is affected by many different factors and processes in water bodies and can fluctuate over short periods of time. Fortunately, most aquatic life can tolerate short periods when oxygen levels are low. However, persistent problems with low oxygen levels can lead to poor aquatic environmental health. For this reason, routine monitoring of oxygen levels is important when there are concerns about the health of aquatic life.
References
[1] American Public Health Association (APHA) (2005) Standard methods for examination of water and wastewater, 21st edn. APHA, AWWA, WPCF, Washington.
[2] FAO. (2014). Site selection for aquaculture: Chemical features of water. Washington, DC: Fisheries and Aquaculture Department, www.fao.org.
[3] U.S. Environmental Protection Agency (1986) Ambient water quality criteria for dissolved oxygen. EPA 440/5-86-003.

